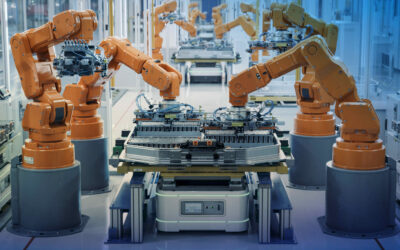
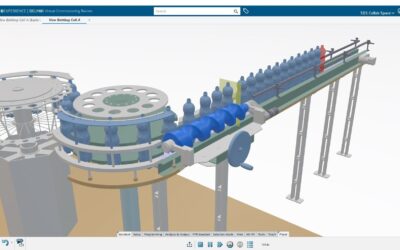
The medical device industry must innovate faster in an increasingly demanding regulated environment, where traceability, risk control, and regulatory compliance are embedded in the product design itself and cannot be managed as isolated or purely documentation-based processes.
Medical devices
Safe innovation in a regulated environment
Connecting design, simulation, quality, and manufacturing within a continuous digital environment makes it possible to reduce risks, avoid nonconformities, and accelerate time to market, transforming regulatory complexity into a sustainable competitive advantage.
Addressing every phase of the device lifecycle
Medical device development requires integrating clinical, regulatory, and user requirements from the start. Requirements management, design control (DHF, DMR), and traceability are critical to avoiding delays and rework.
Multiphysics simulation and virtual validation make it possible to assess performance, safety, and reliability before manufacturing physical prototypes. This reduces costs, shortens cycles, and improves regulatory predictability.
Process planning, synchronization between engineering and production, and controlled execution are essential to ensure “as-designed” and “as-registered” devices, minimizing deviations and recall risks.
Transform regulatory complexity into a competitive advantage
Main challenges:
Regulations are evolving toward a full lifecycle perspective, requiring traceability from concept to clinical use. Reactive management is no longer sufficient; a predictive, data-driven approach is required.
Devices are increasingly personalized, connected, and combined with software and digital services. Integrating clinical requirements, ergonomics, and connectivity increases development complexity.
Manufacturers, suppliers, technology partners, and regulatory bodies form a highly interdependent network. Lack of digital continuity creates errors, delays, and risks of noncompliance.
Market growth and competition — including technology players — require faster innovation without compromising safety or compliance.
Product changes, smaller batch sizes, and greater personalization require flexible processes, virtual validation, and real-time visibility.
Medical device manufacturers need to connect design, requirements, simulation, quality, and manufacturing within a continuous digital environment that ensures full traceability and risk control throughout the entire lifecycle.
As a partner of Dassault Systèmes, CADTECH drives an end-to-end, data-based transformation that reduces nonconformities, anticipates risks, and accelerates the launch of safe and effective products.

Do you want to innovate in medical devices with full control and traceability?
Boost your potential with the solutions that are transforming the industry.
CAD
Product Design
CAE
Product Simulation
CAM
Digital manufacturing
PLM
Project Management
MBSE
Model-based systems engineering
The 3DEXPERIENCE Platform
The 3DEXPERIENCE platform enables unified collaboration throughout the entire product lifecycle, integrating design, simulation, and management within a single digital environment.
It facilitates innovation and optimization at every stage of development for a more agile and competitive product launch. Discover more about 3DEXPERIENCE here.